Introduction to Dissolution
What is Tablet Dissolution?
Tablets or capsules taken orally remain one of the most effective means of treatment available. The effectiveness of such dosage forms relies on the drug dissolving in the fluids of the gastrointestinal tract prior to absorption into the systemic circulation. The rate of dissolution of the tablet or capsule is therefore crucial.
One of the problems facing the pharmaceutical industry is to optimise the amount of drug available to the body, i.e. its bioavailability. Inadequacies in bioavailability can mean that the treatment is ineffective and at worst potentially dangerous (toxic overdose).
Drug release in the human body can be measured in-vivo by measuring the plasma or urine concentrations in the subject concerned. However, there are certain obvious impracticalities involved in employing such techniques on a routine basis. These difficulties have led to the introduction of official in-vitro tests which are now rigorously and comprehensively defined in the respective Pharmacopoeia.
Tablet Dissolution is a standardised method for measuring the rate of drug release from a dosage form. The principle function of the dissolution test may be summarised as follows:
- Optimisation of therapeutic effectiveness during product development and stability assessment.
- Routine assessment of production quality to ensure uniformity between production lots.
- Assessment of ‘bioequivalence’, that is to say, production of the same biological availability from discrete batches of products from one or different manufacturers.
- Prediction of in-vivo availability, i.e. bioavailability (where applicable).
Although initially developed for oral dosage forms, the role of the dissolution test has now been extended to drug release studies on various other forms such as topical and transdermal systems and suppositories.
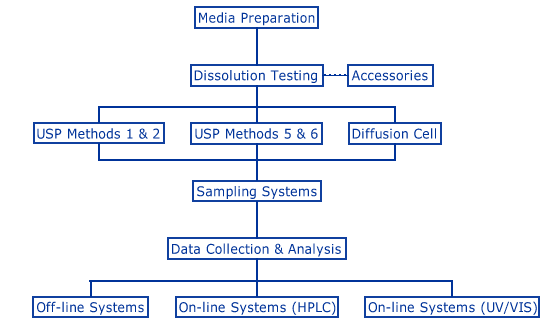
Stages in the dissolution testing process
